Development and challenges of "off-the-shelf" allogeneic CAR-T cells
Nanjing Katy Medicine July 10
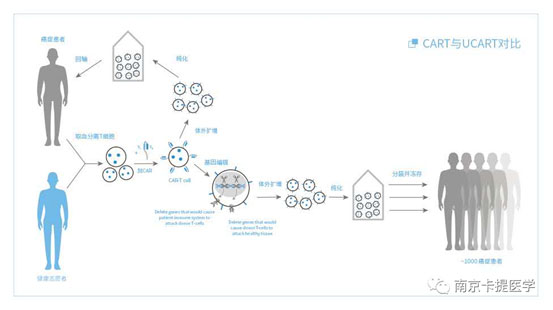
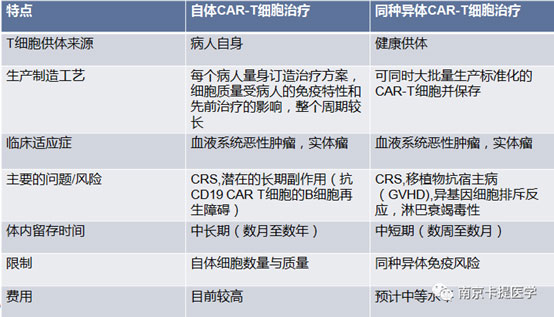
1. Autologous CAR-T cell therapy and allogeneic CAR-T cell therapy
2. Advantages of allogeneic CAR-T cell therapy
Source: From healthy donors, these cells are not affected by the immune effects of cancer, nor are they affected by chemotherapeutics, which are different from the formation of autologous T cells in patients.
Cycle: Compared with autologous CAR-T therapy, allogeneic CAR-T can shorten the treatment cycle, and can use cells that have been prepared in batches for rapid treatment.
Cost: Through standardized preparation of large quantities of CRA-T cells, the cost is greatly reduced.
3. The main problems and solutions in allogeneic CAR-T treatment
Ø Graft versus host disease(GVHD);
Ø Allogeneic T cells may be recognized and eliminated by host cells.
Ø Use allogeneic CART cells from stem cell transplantation donors.
Ø Use virus-specific memory T cells.
Ø Use non-αβ T cells.
Ø Use gene editing methods.
4. Use allogeneic CAR T cells from stem cell transplant donors
T cells can be produced from embryonic stem cells and induced pluripotent stem cells (IPSC). Placenta-derived stem cells can be used to produce T cells or natural killer cells. The placenta has a unique human leukocyte antigen (HLA) expression pattern. Unlike other tissues, the extravillous cytotrophoblast cells only express HLA-C, HLA-E and HLA-G, but the effects of these specificities on placental-derived T cells have not been reported. IPSC can also be a source of CAR-T cells, and an iPSCs library with a common HLA haplotype can be used to reduce the risk of CAR-iPSCT cell allogeneic rejection. One advantage of iPSCs is that CAR T cells are produced by a pluripotent stem cell line and are therefore homogeneous.
5. Use virus-specific memory T cells
Virus-specific T cells are used to treat viral infections. Advances in technology have made it possible to purify memory virus-specific T cells, which can prevent viral diseases without clinical symptoms of GVHD. The adoptive transfer of HLA partially matched virus-specific T cells from healthy blood donors has positive results for Epstein-Barr virus-related malignancies (such as post-transplant lymphoproliferative diseases), with an effective rate of 60-70%, toxic or The incidence of GVHD is low). Therefore, some organizations and biotech companies are developing CART cell lines based on allogeneic Epstein-Barr virus-specific T cell lines.
6. Use non-αβ T cells
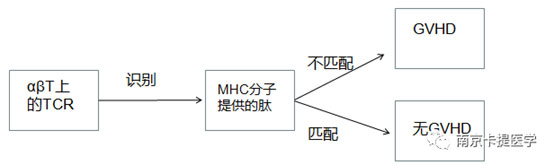
MHC molecule: major histocompatibility complex.
NK cells were originally believed to have the ability to kill tumor cells and are a component of the body's natural tumor immune surveillance. Several studies have shown that NK cell dysfunction in different types of cancers indicates that cancer has evolved a mechanism to escape NK cell killing. Therefore, providing CAR for NK cells to enhance the anti-tumor activity of NK cells is an attractive strategy.
7. Use gene editing methods
Because the TCR fragment of αβT plays a decisive role in the rejection of implants, researchers have developed a method to prevent the expression of functional TCR on the surface of αβT cells, generating specific DNA double-strand breaks at pre-selected positions, while as much as possible Avoid breaks outside the target. Once the DNA break occurs, the cellular DNA repair mechanism will cause gene inactivation (gene knockout) through error-prone non-homologous end joining pathways, or lead to gene insertion or correction (gene knockout) through homologous recombination pathways. The first reported study evaluated the feasibility of knocking out TRAC in CART cells. The authors of the report pointed out that the elimination of TCR expression through zinc finger technology does not impair the anti-tumor properties of CD19-specific CART cells.
8. Challenges facing allogeneic CAR T cell therapy
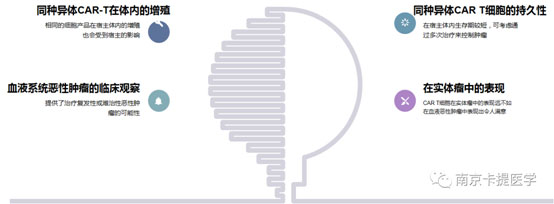
9. Proliferation of allogeneic CAR-T in vivo
After long-term research, it has been determined that lymphatic wasting chemotherapy is necessary for effective T cell expansion in vivo. After T cells are transferred to a host with lymphopenia, they undergo a process called steady-state expansion. This expansion is driven by steady-state cytokines such as IL-7 and IL-15 and exposure to self-antigens and other antigens. The best pretreatment plan has not been determined. The lack of TCR expression in allogeneic CAR-T cells using gene knockout methods may be an advantage, because TCR participation in combination with CAR activation will have a negative impact on the expansion of CD8+CART cells. However, the above conclusions are drawn in mouse models, and more clinical data are needed.
10. Persistence of allogeneic CAR T cells
The survival cycle of allogeneic CAR-T cells in vivo is relatively short, but depending on the nature of the disease, tumor burden and other factors, the optimal duration of CART cell survival may be different. Two situations can be considered.
Most tumor cells expressing target antigens can be utilized by CART cells. If a sufficient ratio of effector cells to target cells can be obtained after expansion, then there is no need to maintain long-term persistence after tumor cells are eliminated. 2. In the second case, it may be more common that tumor cell populations that are less sensitive to T cell killing continue to complete clinical remission. In this case, it is necessary to envisage long-term control of residual disease through CARTT cells. It is conceivable that after the first administration, several more administrations of allogeneic CAR-T. The limitation of this approach is that lymphatic clearance must be performed before each administration, and the intensity of lymphatic clearance and the number of administrations need to be limited.
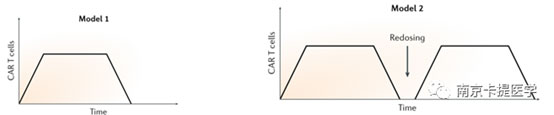
11. Clinical observation of hematological malignancies
Allogeneic CART cells offer the possibility of treating relapsed or refractory malignancies, which may be a major advantage for rapidly progressing diseases such as AML and ALL. Trials for ALL and AML have already begun, and trials are planned for lymphoma and multiple myeloma. The target is similar to the autologous pathway, including CD19 and CD22 in ALL and B-cell lymphoma, CD30 in Hodgkin’s lymphoma and anaplastic large cell lymphoma, and B-cell maturation protein (BCMA, also in multiple myeloma) It is called TNFRSF17), CS1 (also called SLAMF7) and CD38, and CD123. For targets expressed by normal cells like CD123, the shorter duration of allogeneic CART cells may be an advantage.
12. Performance in solid tumors
The performance of CART cells in solid tumors is far less satisfactory than that in hematological malignancies. Inducing more powerful CAR-T cells to deal with the microenvironment and oxidative stress of solid tumors is a good research direction. There are currently several strategies to make CAR-T cells resist the tumor microenvironment.
Make CAR-T cells resist the tumor microenvironment. CAR T cells expressing catalase maintain their anti-tumor activity under H2O2-induced oxidative stress.
Use multiple steps of gene editing to optimize CAR-T cell function. Some people have proposed some strategies to reduce the sensitivity of T cells to negative immune checkpoints and immunosuppression.
Combining multiple signals from the tumor microenvironment to activate T cells in the tumor. T cells constructed using a three-component split CAR system, whose inner domain reproduces physiological T cell signals by transmitting signal 1 (activated by CD3ζ), signal 2 (co-stimulation by 4-1BB) and signal 3 (cytokine release) (IL-7)).
13. Conclusion
CAR-T cell therapy is one of the most promising methods for the treatment of cancer. On this basis, general-purpose CAR-T cells are developed to reduce costs and allow more people to use this therapy. And new technologies have been developed to make the host's immune system less responsive or even "invisible" to allogeneic AR-T cells. Although there are still many challenges in improving the efficacy of CAR-T, especially in solid tumors, there are still many ways to achieve optimization.